What is Climedo Health?
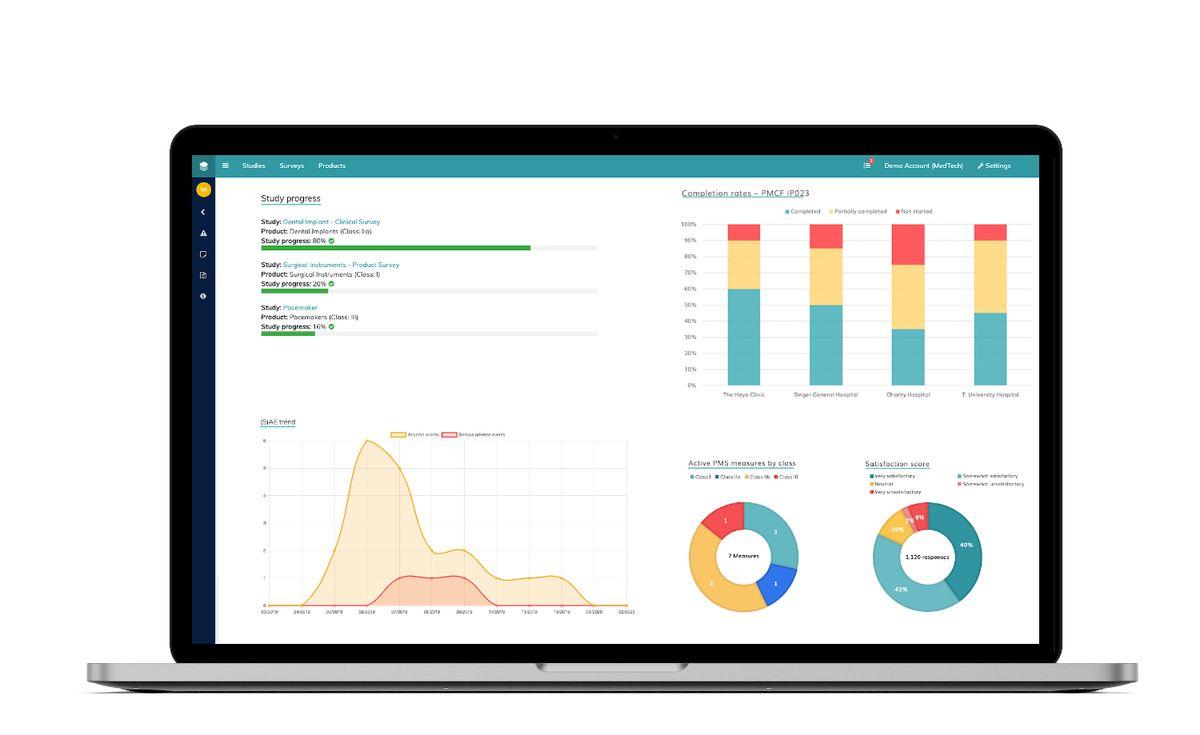
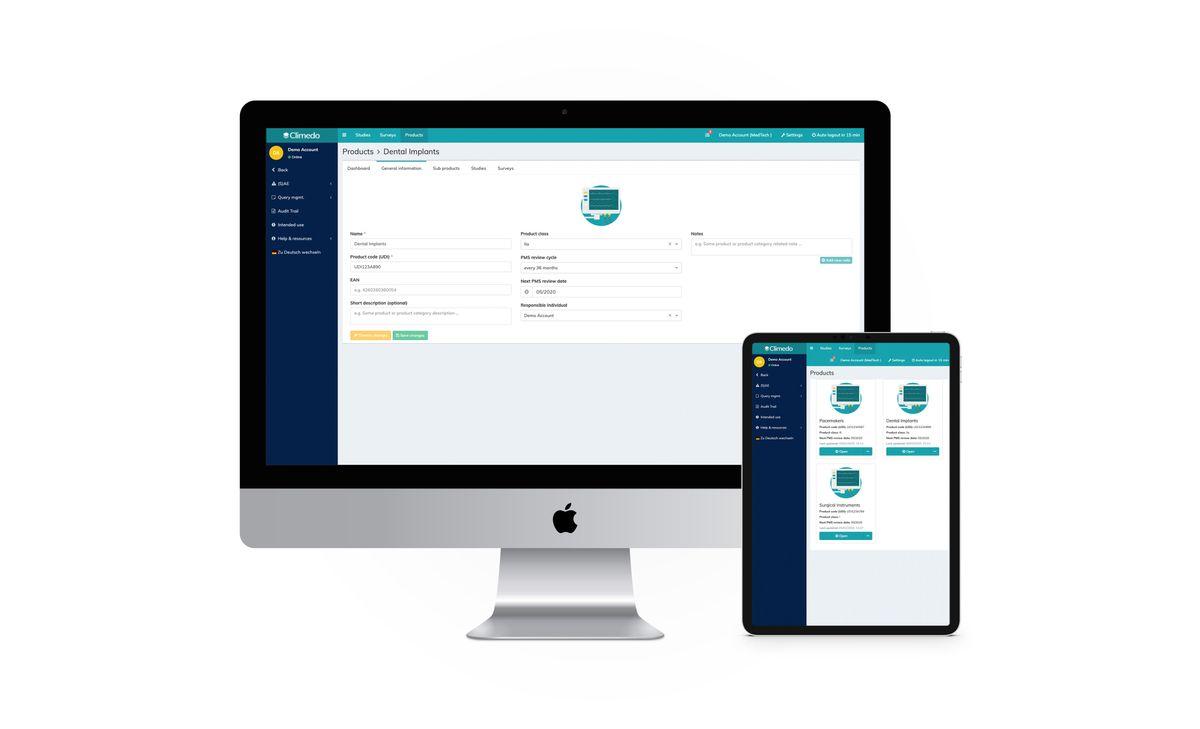
Climedo's cloud-based platform provides a comprehensive electronic data capture (EDC) solution for the validation and post-market surveillance of medical devices, in line with the EU Medical Device Regulation (MDR). This secure and cost-efficient platform consolidates all aspects of clinical data collection, connecting all stakeholders involved in the research process, including medical device manufacturers, pharmaceutical companies, contract research organizations (CROs), hospitals, and patients
Highlights
- ePRO (electronic patient-reported outcomes) and eCOA (electronic clinical outcome assessments) capabilities
- eDiaries and eSurveys for flexible data capture
- Seamless integration with other health information systems through APIs
- Fast setup and intuitive user interface with minimal training required
- Significant cost savings, up to 50 in documentation effort and 80 in onboarding time
- Compliance with the strict data protection laws of Germany, ensuring data security
Features
Product Catalog Manager
Multiple languages
Cloud based
Survey & feedbacks
Browser-based
Intuitive
QR Code Generator
Audit Trail